INTRODUCTION
Antibiotic resistance caused by antibiotic abuse poses a serious threat to human health. The antibiotic resistance rate in China is gradually increasing, especially in primary medical institutions. Almost half of the prescriptions in rural areas of western China include antibiotics1-3. Studies have shown that by 2050, 10 million people worldwide will be killed from drug-resistant infections4, and the rational use of antibiotics has attracted the attention worldwide. As early as 2009, China issued the ‘Opinions on Deepening the Reform of the Medical and Health System’ and the ‘Notice on Issues Related to the Clinical Application Management of Antimicrobial Drugs’, which clearly stipulates strict control and regulation of the use of antibacterial drugs. After the introduction of these policies, the use of antibiotics showed a differential change5. Primary medical institutions are an important part of China’s healthcare work and the main part of poor antibiotic use3,5. We used grassroots doctors as the main group to collect and analyze the questionnaires in five counties (cities) of Sichuan Province, China, from August 2018 to March 2019. We examined the use cognition status quo of antibiotics after ten years of the ‘new medical reform’ and analyzed the impact of the implementation of relevant policies and drug systems on primary doctors. We provide recommendations for promoting the rational use of antibiotics.
METHODS
Research objects
From August 2018 to March 2019, five counties (cities) were randomly selected according to the level of urban development and geographical distribution in Sichuan Province, China. Each county and city randomly selected 1 first-level hospital, 3 community hospitals, 3 township hospitals, and 5 village clinics and private enterprises. Each level 1 hospital had 10 to 20 doctors, covering all departments; each community hospital and township hospital investigated about 10 doctors; each village clinic and private clinic investigated 1 or 2 doctors. We sent out a questionnaire to each doctor. A total of 430 valid questionnaires were collected, including 88 first-class hospitals, 129 community hospitals, 130 township health centers, 41 village clinics, and 42 private clinics.
Questionnaire investigation
We design the status quo of antibiotic use and cognitive questionnaires of the doctors at the grassroots level. In the questionnaire description, it was clarified that the research was only used for academic research and was filled in anonymously. All the respondents in this study signed the informed consent form. The researcher collected the audit and summarized the analysis. According to the contents of the doctor’s questionnaire, we used Excel 2016 to organize the database. The survey included basic information (Table 1), antibiotic-related cognition, current status, and some relevant views on current antibiotic abuse. The questionnaire involves several common topics in clinical treatment (Table 2), and there are 7 knowledge questions and 1 attitude question in the cognitive part of the questionnaire (Table 3), with 3 options ‘Yes’, ‘No’ and ‘Unclear’, with a score of 1 point if the answer was correct (correct answers to these topics come from representative literature6-9), and 0 points for incorrect answers or ‘not known’.
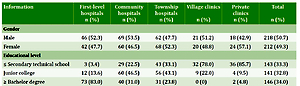
Table 1
Basic information of doctors in various primary medical institutions (N=430)
Table 2
Comparison of antibiotic use among doctors in primary health care institutions (N=430)
| *Variables | First-level hospitals n (%) | Community hospitals n (%) | Township hospitals n (%) | Village clinics n (%) | Private clinics n (%) | Total n (%) | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| Drug use authority | 39.889 | <0.001 | ||||||
| Understand | 88 (100) | 121 (93.8) | 124 (95.4) | 31 (75.6) | 32 (76.2) | 396 (92.1) | ||
| Do not understand | 0 (0) | 8 (6.2) | 6 (4.6) | 10 (24.4) | 10 (23.8) | 34 (7.9) | ||
| Preference of antimicrobial spectrum | 18.743 | <0.001 | ||||||
| Broad spectrum | 5 (5.7) | 16 (12.4) | 23 (17.7) | 8 (19.5) | 14 (33.3) | 66 (15.3) | ||
| Narrow spectrum | 83 (94.3) | 113 (87.6) | 107 (82.3) | 33 (80.5) | 28 (66.7) | 364 (84.7) | ||
| Preference of administration mode | 34.217 | <0.001 | ||||||
| Oral administration | 5 (5.7) | 7 (5.4) | 28 (21.5) | 4 (9.8) | 5 (11.9) | 49 (11.4) | ||
| Injection | 26 (29.5) | 16 (12.4) | 16 (12.3) | 10 (24.4) | 9 (21.4) | 77 (17.9) | ||
| Not always | 57 (64.8) | 106 (82.2) | 86 (66.2) | 27 (65.9) | 28 (66.7) | 304 (70.7) | ||
| Preventive use of antibiotics | 20.259 | <0.001 | ||||||
| Yes | 28 (31.8) | 26 (20.2) | 25 (19.2) | 16 (39.0) | 20 (47.6) | 115 (26.7) | ||
| No | 60 (68.2) | 103 (79.8) | 105 (80.8) | 25 (61.0) | 22 (52.4) | 315 (73.3) | ||
| Combined antibiotic | 2.773 | 0.597 | ||||||
| Yes | 29 (33.0) | 46 (35.7) | 40 (30.8) | 18 (43.9) | 13 (31.0) | 146 (33.9) | ||
| No | 59 (67.0) | 83 (64.3) | 90 (69.2) | 23 (56.1) | 29 (69.0) | 284 (66.1) | ||
| Empirical medication | 17.284 | <0.05 | ||||||
| Yes | 74 (84.1) | 110 (85.3) | 124 (95.4) | 39 (95.1) | 42 (100) | 389 (90.5) | ||
| No | 14 (15.9) | 19 (14.7) | 6 (4.6) | 2 (4.9) | 0 (0) | 41 (9.5) | ||
| Drug prescription in the face of patient pressure | 21.324 | <0.05 | ||||||
| Open | 20 (22.7) | 22 (17.1) | 11 (8.5) | 3 (7.3) | 13 (31.0) | 69 (16.1) | ||
| Not open | 51 (58.0) | 81 (62.8) | 97 (74.6) | 29 (70.7) | 19 (45.2) | 277 (64.4) | ||
| Not always | 17 (19.3) | 26 (20.2) | 22 (16.9) | 9 (22.0) | 10 (23.8) | 84 (19.5) | ||
| Relevant guidance | 8.801 | 0.066 | ||||||
| Comply | 53 (60.2) | 66 (51.2) | 85 (65.4) | 21 (51.2) | 19 (45.2) | 244 (56.7) | ||
| Non-compliance | 35 (39.8) | 63 (48.8) | 45 (34.6) | 20 (48.8) | 23 (54.8) | 186 (43.3) | ||
| Relevant institutional impacts | 56.424 | <0.001 | ||||||
| Yes | 76 (86.4) | 107 (83.0) | 107 (82.3) | 24 (58.5) | 15 (35.7) | 329 (76.5) | ||
| No | 12 (13.6) | 22 (17.0) | 23 (17.7) | 17 (41.5) | 27 (64.3) | 101 (23.5) | ||
Table 3
Comparison of cognitive correctness rates and attitudes of doctors in various primary health care institutions
| *Topic (correct answer) | First-level hospitals % | Community hospitals % | Township hospitals % | Village clinics % | Private clinics % | Total % |
|---|---|---|---|---|---|---|
| Antibiotics are antiinflammatory drugs (no) | 98.90 | 66.70 | 68.50 | 53.70 | 59.50 | 71.90 |
| Patients should discontinue antibiotics as soon as their symptoms improve (no) | 78.40 | 71.30 | 77.70 | 58.50 | 47.60 | 71.20 |
| Antibiotics are the first choice for influenza and common cold (no) | 93.20 | 68.20 | 71.50 | 61.00 | 52.40 | 72.10 |
| Antibiotics are effective for most acute upper respiratory infections (no) | 73.90 | 69.00 | 67.70 | 70.70 | 64.30 | 69.30 |
| Overuse of antibiotics can lead to bacterial resistance (yes) | 100.0 | 95.40 | 97.70 | 95.10 | 88.10 | 96.30 |
| The more expensive the antibiotics, the better the efficacy (no) | 81.80 | 69.80 | 78.50 | 56.10 | 42.90 | 70.90 |
| Antibiotics can be taken orally but not injected (yes) | 88.60 | 86.10 | 90.80 | 80.50 | 69.10 | 85.80 |
| My knowledge of antibiotics needs to be improved | 38.60 | 84.50 | 73.90 | 80.50 | 95.20 | 72.60 |
Statistical analysis
SPSS 25.0 was used to describe and analyze the data. The comparison between doctors in various types of primary medical institutions was performed by a χ2-test. The differences in antibiotic-related cognitive scores of different groups under different demographic characteristics were tested by Wilcoxon rank-sum test. Inspection standards were based on α= 0.05.
Definitions
The ‘drug use authority’ is the State Drug Administration, China. The ‘preventive use of antibiotics’ is ‘the use of antibiotics before the onset of a disease to prevent possible infection in a specific population’. The ‘combined antibiotics’ is using two or more types of antibiotics. The ‘empirical medication’ is the practice of using antibiotics based on the clinical experience of a doctor before identifying which pathogens cause the infection. The ‘relevant compliance’ is the Guidelines for Clinical Application of Antimicrobial Drugs issued by the Chinese government in 2018. The ‘relevant institutional impacts’ refer to National Health Commission of the People’s Republic of China (NHC), subordinate units of NHC and hospitals where doctors work.
RESULTS
Basic information of the respondents
Among the 430 doctors, 218 were males with an average age of 43.3 years and 212 females with an average age of 35.8 years. With regard to the educational level, secondary school (high school) and below accounted for 33.3%, college graduates were 32.8%, and undergraduate and above were 34.0%. Those who entered the profession after the ‘new medical reform’, i.e. whose work experience was less than 10 years accounted for 37.2%; those who entered the profession before the ‘new medical reform’, i.e. whose work experience was 10–20 years accounted for 35.4% ; those in the top 10 of the ‘new medical reform’, i.e. more than 20 years of work experience accounted for 27.4%. A total of 359 people (83.5%) had relevant learning and training experience, with the highest level in the first-level hospitals, reaching 95.5%; the lowest in private clinics, being only 42.9% (Table 1). The training organization mainly includes county and city health offices, county and city hospitals, disease control centers and medical schools.
Comparison of current status of antibiotic use among doctors in different primary medical institutions
There was no significant difference in the level of understanding of antibiotic use among village clinics and private clinic doctors (p=0.95), but they were both lower than those of county, township and community hospitals; a small number of doctors preferred broad-spectrum antibiotics, mostly those from private clinics, 14 (33.3%); while 26.7% of doctors preferred preventive use of antibiotics; 34.0% had combined use of different types of antibiotics (mainly for inpatients), the difference was not statistically significant (p=0.60), but for the village clinics this was higher (43.9%).
Empirical medication was quite common (90.5%), and the difference was statistically significant (p<0.05). It is worth mentioning that empirical medication was mostly in township hospitals, village clinics and private clinics, the difference was not statistically significant (p=0.36). When faced with the pressure of patients to use antibiotics, first-level hospitals and private clinic doctors were persuaded the highest (22.7% and 31.0%, respectively); only 56.7% of the doctors were able to comply with guidance on the use of antibiotics; but generally, the compliance rate of doctors in all levels of hospitals was low, the difference was not statistically significant (p=0.07); only 76.5% of doctors indicated that the implementation of the relevant drug system has affected the use of antibiotics, private clinic doctors had the least impact (35.7%) while the first-level hospital doctors had a relatively large impact (86.4%).
Comparison of antibiotic use among doctors in primary health care institutions
Statistics show that the overuse of antibiotics led to the highest awareness of bacterial resistance, the correct rate was 96.3%; the antibiotics were the worst for most acute sensations, and only 69.3% of the respondents answered correctly. Doctors that realized their knowledge of antibiotics needed to be improved had the lowest rate in first-level hospitals (38.6%) and highest in private clinics (95.2%), shown in Table 3. The median number of antibiotic-related cognitive scores was 6, and the IQR was 5–6, indicating that doctors have better knowledge of antibiotics. Because the knowledge scores were skewed, we used Wilcoxon rank-sum test to analyze whether the scores of the different groups are different for different demographic characteristics. The rank sum-test shows that the cognitive differences of doctors with or without relevant training experience are statistically significant (p=0.018), those with the relevant training were better than the untrained doctors; the cognitive differences of doctors with different working years were statistically significant (p=0.038), and the doctors working under 10 years were better than those with 10–20 working years. There was no significant difference in the correct rate of antibiotic use among doctors with different academic qualifications (p=0.077). There was no significant difference in knowing the correct rate of antibiotic use among doctors of different genders (p=0.076).
Analysis of the reasons for the non-standard use of antibiotics by doctors at the grassroots level
Through open-ended questionnaire investigation, we learned that there were three basic sources for unreasonable use of antibiotics by grassroots doctors. First, limited by laboratory technology and diagnostic techniques, primary hospitals often use antibiotics prophylactically and empirically; their understanding of antibiotic application is not comprehensive; their wages are low, especially in private clinics, which are vulnerable to economic interests; doctors and patients are nervous, want to quickly achieve the desired therapeutic effect resulting in antibiotic abuse. Second, patients have high expectations for treatment, often judge the doctor’s level by the patient’s disease condition, driving them to abuse antibiotics; the patient’s awareness of the rational use of antibiotics was not strong, and the risks were not well understood. Often the patients blindly asked the doctor to prescribe antibiotics, and when they could not obtain a prescription they would buy antibiotics themselves. Third, the relevant institutions are not strict with the regulation of supply and marketing channels for antibiotics. Many patients have purchased antibiotics through e-commerce platforms. The convenience of people purchasing antibiotics is as simple as buying ordinary items online; the relevant health system departments insufficiently inform medical staff in village clinics, private clinics and pharmacies about antibiotics.
DISCUSSION
We believe that the knowledge of antibiotic use among doctors in primary healthcare institutions needs to be strengthened. It is also necessary to increase the training on rational use of antibiotics, especially for doctors in village and private clinics. Their rate of understanding of correct antibiotic use is less than 80%. Some grassroots doctors still think that antibiotics are anti-inflammatory drugs, often use antibiotics prophylactically, do not know or do not implement the principle of antibiotics use, which is that antibiotics should be taken orally and not injected, and even then they should be administered by intramuscular injection instead of intravenously9. Some doctors still think that antibiotics are used to treat influenza and that they are the common choice for the cold, leading to an increase in the rate of drug use. The proportion of preventive, empiric, and combined use of antibiotics in village clinics and private clinics is higher than in first-level hospitals, community hospitals and township hospitals. This may be related to training experience, awareness of correct drug use, and compliance with relevant institutional guidelines. Firstlevel hospitals and private clinics have the highest rate of antibiotics use; first-level hospitals because of the large number of patients, the complexity of patients’ conditions and the relationship between patients and doctors; in private clinics the reasons may be related to factors such as profit and marketing. Doctor prescriptions are largely influenced by doctor-patient relationships and patient expectations10-11, but patient expectations are often based on the attitude of doctors12, so changing doctors’ unreasonable antibiotic use behavior is vital to solve this problem. Regarding the use of antibiotics, doctors with the relevant training are better than the untrained, and those with more than 10 years’ experience are better than those with less than 10 years, indicating the implementation of the ‘new medical reform’ policy and the antibiotic-related drug system has had a positive effect on grassroots doctors. It is still necessary to strengthen the implementation level and maximize its effectiveness.
Unreasonable use of antibiotics is affected by many factors such as doctors, patients, and society. We propose the following improvements. Regulators and hospitals need targeted training programs to promote doctors’ understanding of antibiotics13, timely follow-up on the latest medication guidelines, and comprehensively grasp the indications for rational use of antibiotics. Relevant departments must strengthen supervision, improve antibiotic production, sales, use mechanisms, standardize antibiotic prescription behaviors of grassroots doctors, focus on e-commerce platform, private clinics and pharmacies that are the sale channels. Improve the salary of medical staff, strictly enforce relevant laws and regulations, and curb rebates. Improve the medical environment, ease the contradiction between doctors and patients, educate about the harm of antibiotics abuse, and change the public’s incorrect understanding and use of antibacterial drugs14. In addition, encourage innovation cooperation, research on new antibiotic production, rapid diagnosis of clinical pathogens15, establish a comprehensive, highly responsive antibiotic use and drug-resistant infection monitoring system, implement clinical drug guidelines, regulate professionals and public to the medicine Act, participate in international cooperation, and build a platform for the transformation and cooperation of scientific research results16-18. Also, encourage joint detection of pathogens in various regions, clarify the epidemiological characteristics of pathogens in various regions, and improve theoretical guidance for empirical drug use.
CONCLUSIONS
Ten years after the introduction of the new healthcare reform policy, the improvement of antibiotic use is still far from the expected goal; the knowledge of antibiotic rational use of doctors in primary medical institutions needs to be strengthened, especially in village clinics and private clinics; the joint efforts of doctors, patients and supervisors are needed to promote the rational use of antibiotics.